- Stereoisomerism ( Space isomerism)
stereoisomerism is the isomerism which arises due to a different arrangement of atoms or molecules in space so, they are also called space isomerism. They have the same structural formula but different configuration.
- Geometrical isomerism:
It arises due to the different geometrical arrangement of atoms or group of the atom around the rigid bond about which group of an atom is to distributed is called geometrical isomerism. It is also known as cis-trans isomerism.
Cis-isomerism: If the similar groups are on the same side of a rigid bond then this type of isomerism is called cis-isomerism.
Trans-isomerism: If the different groups are on the same side of a rigid bond then this type of isomerism is called trans-isomerism.
2. Optical isomerism:
Those compound which shows similar chemical reaction as well as physical properties but shows different rotation towards the plane polarised light such substances are called optical isomers and the phenomenon is known as optical isomerism. Optical isomerism is of two types i.e. enantiomerism and diastereoisomerism.
Enantiomerism: The optically active compound which is a non-super-imposable mirror image to each other. They rotate the path of plane polarised light to the same extent but in opposite direction.
Diastereoisomerism: Those optical isomers which do not have mirror images to each other are called diastereomers. The phenomenon is called diastereoisomerism.
Optical Activity: It is the rotation of the plane of plane-polarized light either left (laevo) or right(dextro) is called optical activity and the compound which shows optical activity are called an optically active compound. e.g. Generally chiral compound shows optical activity such as Lactic acid, Glyceraldehyde, Glucose, etc.
Meso compounds:
Meso compound contains the multiple chiral centres which are a mirror image of one another. Meso compound contains the internal mirror image that means one half is the mirror image of another ( contains a line of symmetry). If the compound doesn't have the line of symmetry then it is not meso compound. The line of symmetry is the basic property of meso compound.
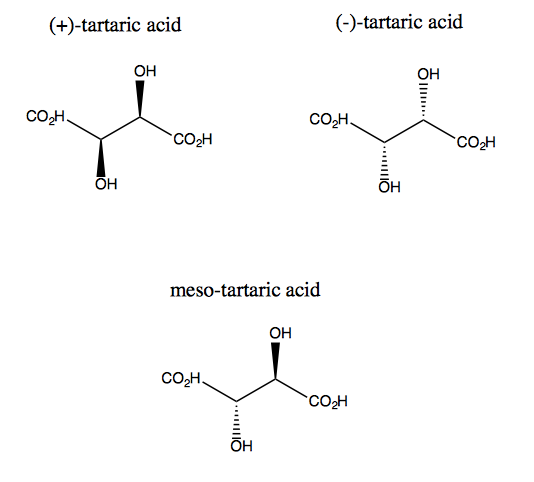
In above meso-tartaric acid left and right sides are a mirror of each other and are symmetric about the internal mirror plane. In meso tartaric acid one half is completely overlapped with another half so, they don't show optical activity. The rotation of one half cancels the rotation of another half ( Internal compensation).
meso tartaric is optically inactive due to internal compensation.

Helped me a lot during studying
ReplyDeletethank you
Deleteoutstanding
ReplyDeleteThank you
ReplyDeleteThank you
ReplyDeleteGreat friend keep it up
ReplyDeleteThanks bro
DeleteGood job bro. Keep doing this .
ReplyDeleteoutstanding
ReplyDeletegreat brother
ReplyDeleteawesome
ReplyDeleteGreat bhaiii ji...
ReplyDelete